length of a standard autoclave run|autoclave cycle requirements : broker Prior to operating an autoclave, all laboratory personnel must complete training from their supervisor on standard autoclave working procedure. Training materials should . See more Acheter la lame de terrasse en pin sylvestre autoclave classe 4 c’est choisir le meilleur rapport qualité prix ! Le traitement autoclave classe 4 lui procure une protection contre la pouriture et .
{plog:ftitle_list}
Rio Tinto, Kennecott Utah Copper’s (KUC) parent company, will invest $340 million in the construction of a new molybdenum autoclave process (MAP) facility at KUC in Magna, .
To ensure that procedures are followed correctly, maintain the safety of laboratory staff, and increase the likelihood that sterilization is achieved with no errors, having a set of autoclave SOPs is crucial. By establishing these types of guidelines, all technicians (or anyone who uses your lab’s sterilization . See more
Prior to operating an autoclave, all laboratory personnel must complete training from their supervisor on standard autoclave working procedure. Training materials should . See moreIt’s important that laboratory personnel never place sealed containers in the autoclave, as the pressure inside the autoclave could cause glassware to crack or explode. Likewise, . See more
When loading, operating, or unloading an autoclave, laboratory personnel must wear the following: 1. Close-toed shoes 2. Safety glasses 3. Heat-resistant gloves that completely cover . See moreLaboratory personnel should always observe the following autoclave procedures when loading the unit: 1. Loosen the lids on . See moreRun the autoclave at a chamber temperature of 121°C for 60 minutes*, using a dry cycle run. .• Run the autoclave at a chamber temperature of 121°C for 60 minutes *, using a dry cycle run. * 121°C is a standard temperature for autoclave operation, and generally achieved when chamber pressure is 15-16 psi. However, this pressure is dependent upon altitude. At higher altitudes, the pressure must be increased to achieve 121°C. It is .
Sterilization monitoring is necessary for each autoclave load, including mechanical and chemical indicators as required and recommended, plus, depending on your location, spore testing (biological indicators). Spore testing . The gravity displacement autoclaves are primarily used to process laboratory media, water, pharmaceutical products, regulated medical waste, and nonporous articles whose surfaces have direct steam contact. . the front, bottom section of the sterilizer rack, near the door and over the drain, in an otherwise empty chamber and run at 134°C for .Autoclave manufacturers generally provide several pre-set cycle options that vary in the pre-set sterilization temperature, sterilization time and dry time. . After a run is complete, check the pressure gauge to ensure that the pressure in the chamber is “0.” If pressure is not released, do not open the door. .
j mitra dengue elisa kit
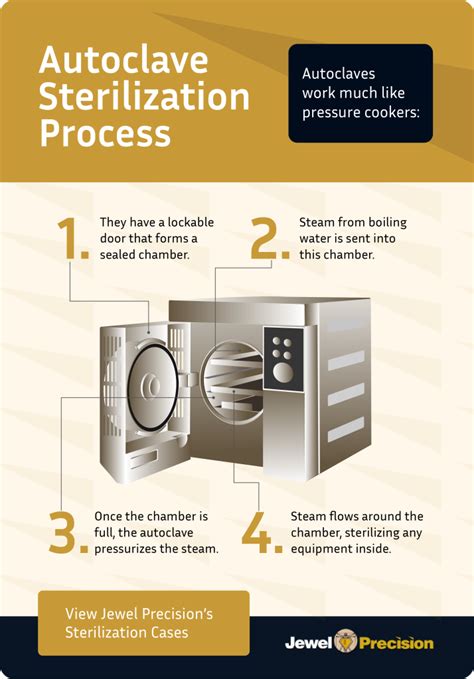
How long do I autoclave? Steris provides a table which can be used as a roadmap for your liquid autoclave runs. • Guidelines are in terms of “time” vs. “volume per flask”. • Suggested sterilization times are for “water-like” liquids in bottles or flasks. Media with additives may need to have shorter times so that it is not .Note: the indicators only verify that the autoclave has reached normal operating temperatures; they do not indicate that the contents were heated for the appropriate length of time or at the proper pressure. Therefore, tape indicators cannot be used to prove organisms are actually killed during an autoclave run.
From glassware to redbag waste, Consolidated’s sterilizers can run the necessary cycles to accurately sterilize your specific loads. Use the table and graphical information below to learn more about our basic and advanced cycle types, specific applications, sterilization cycle phases, and critical cycle parameters.
How long do I sterilize? Recently, it was reported that media was being burned during the sterilization process. One of the common misconceptions is that sterilization time is not affected by the combination of individual container volumes, total number of containers in autoclave, and how these containers are arranged. Previous information posted about .Alfa Medical Equipment 265 Post Ave Westbury NY 11590 Phone: 1-800-801-9934The length of any autoclave cycle is determined by the size of the autoclave, what the autoclave is sterilizing or its contents, temperature, and what the cycle is supposed to be doing. . So, a lower temperature cycle will run longer than a higher temperature one if all other things are equal. So, to answer the question in the title of this .If growth is noted on the autoclaved spore strips, try increasing the run time. If growth still occurs with run times of 45 minute or more, the autoclave may need maintenance and repair. Autoclave performance information: . and length of time the load is sterilized. . Identification of standard treatment containers and proper load placement .
standard autoclave procedure instructions
Standard Test Method for Autoclave Expansion of Hydraulic Cement1 This standard is issued under the fixed designation C 151/C 151M; the number immediately following the designation indicates the year . ing length change of specimens shall conform to the require-ments of Practice C 490. 6. Temperature and HumidityStudy with Quizlet and memorize flashcards containing terms like Define autoclave, What do autoclaves efficiently sterilize?, What is a subsitute for autoclaves? and more. . Length of time varies, check manufacturer's instructions. Where should sterilized items be placed? Clean and dustproof areas. How long do items remain sterile after .AUTOCLAVE LOG Use of this form is voluntary and not required by the Department of Health. The form is provided as a service to assist salons in complying with the record-keeping requirements of Chapter 64E-19, FAC. DATE LENGTH OF RUN CUMULATIVE TODAY TOTAL CHECK IF CLEANING DONE CHECK IF SERVICING DONE CHECK IF SPORE TESTING .
Standard Operating Procedure for Performance Verification of Autoclaves SOP Number: QC-13-07 Date Revised: 6-15-15. SOP No. QC-13-07 Date Revised 06-15-15 . Autoclave Runs (Per Run Verifications). a. The following data are collected for every autoclave cycle. i. Autoclave Printout. For each run, record the minimum andend of the run. Hot fluid scalds from boiling liquids and spillage in the autoclave. . Read and understand the ASU Autoclave Standard Operating Procedure (SOP). . conditions such as length of cycle(s) and pressure. Autoclave tape works by changing color after exposure to temperatures commonly . EH&S September 2016 Contact Information: (480 . Autoclave sterility monitoring must be conducted at least monthly using appropriate biological indicators (Bacillus stearothermophilus spore strips) placed at locations throughout the autoclave. The spores, which can survive 250°F for 5 minutes but are killed at 250°F in 13 minutes, are more resistant to heat than most, thereby providing an .
The standard pressure is typically 15 psi, which corresponds to a temperature of 121°C. . Cost-effective: Autoclaves are economical in the long run. They eliminate the need for additional chemicals or disposable items, reducing ongoing costs associated with sterilization. . The length of time that autoclaved items will remain sterile will . Here are some key considerations for optimizing autoclave sterilization parameters: Calibration: Regular calibration of autoclave equipment is essential to ensure accurate temperature and pressure settings and consistent sterilization performance. Periodic testing with biological indicators (e.g., spore strips) can verify the effectiveness of .
Autoclave Log: An autoclave log containing the following details should be maintained by lab staff: Date, time, and operator’s name; Contact information: Laboratory, room number, phone number; Type of material sterilized/cycle; Temperature, pressure, and length of time the load is sterilized. Biological Indicator Test Results
The autoclave cycle length is generally given for various materials as a recommended minimum run time by the manufacturer. . Some autoclaves can run cycles at higher temperatures at 132°C or .To be effective, the autoclave must reach and maintain a temperature of 121° C for at least 30 minutes by using saturated steam under at least 15 psi of pressure. Increased cycle time may be necessary depending upon the make-up and volume of the load. The rate of exhaust will depend upon the nature of the load. Dry material can be treated in a fast (Adding a vacuum pump offers other advantages, including the ability to run cycles with a “post-cycle vacuum” stage, which results in much dryer loads.) . Every Priorclave autoclave comes standard with our advanced fully programmable control system, Biomaster® protected antimicrobial surface coatings, free lifetime technical support, and .
je igm elisa kit

Autoclave sterilization units should be tested weekly to determine the function and efficiency of the unit. A biological test containing two test strips is used during this process. Both strips contain highly resistant spores from the same lot. There is the test, the one that gets ran through the sterilizer unit, and the control, the one that .Autoclave Safe Use BSOP-02 Standard Operating Procedure Approved by: IBC Date: Feb 23 2015 BSOP02 v1 Date of first issue: Feb 2015 Document is uncontrolled when printed . Ensure that temperature and pressure settings for the autoclave run were met and that the gauges are at zero, stand back from the door and crack it open slightly, to allow .A new user has to demonstrate knowledge and autonomy prior being authorized to load and run the autoclave alone. 5.3 Operation Manual and resources . Microsoft Word - SOP-MEAK-9001 Autoclave Standard Operational Procedure.docx Author: severine.audusseau Created Date: 9/13/2018 10:46:51 AM .reference standard. For example, an autoclave’s chamber temperature probe’s response can be compared to a previously calibrated device, or “standard”, . So, if you run cycles at three different temperatures (e.g. 115°C, 121°C, and 134°C), you would make calibration measurements at four points (e.g. 110°C, 120°C, 130°C, and 140°C.
standard autoclave manual pdf
autoclave unloading requirements
autoclave sterilization time chart
APPENDIX 6 VACUUM TEST Use this test to validate the performance of the sterilizer in terms of leakage. During the test the following is checked: Efficiency of the vacuum pump. Pressure .
length of a standard autoclave run|autoclave cycle requirements